Introduction: Despite advances in the treatment of hematologic malignancies (HM), a significant number of patients still present with high burden of symptoms, and many still die from their diseases. Adequate integration of palliative care (PC) shows strong potential to improve quality of life of this population. However, PC strategies are underutilized due to several barriers in the context of high prognosis uncertainty. Therefore, it is crucial to implement practical tools and triggers for opportune discussions concerning goals of care and specialist PC consultations. The “Surprise-Question” (SQ) “Would you be surprised if this patient died in the next 12 months?” is a simple and feasible instrument for intuitive estimation of mortality among patients with different types of advanced disease with the aim to improve prognostic awareness among healthcare professionals. In 2016 this instrument was introduced in the American Society of Clinical Oncology (ASCO) Integration of Palliative Care Into Standard Oncology Care Guideline. Most studies refer the SQ to solid tumors and very little is known about the application of this tool in the context of HM. We evaluated the prognostic performance of the SQ among patients with HM.
Methods: Prospective cohort study conducted from August to December 2021 through the application of interviews with hematologists from the Federal University of Bahia Clinics Hospital (HUPES-UFBA). Adult patients (age≥18) with HM admitted to the Hematology ward of Clinics Hospital during the study period were included and the SQ was answered by the physicians responsible for the patients at the time of inclusion. Clinical information was extracted from medical records.
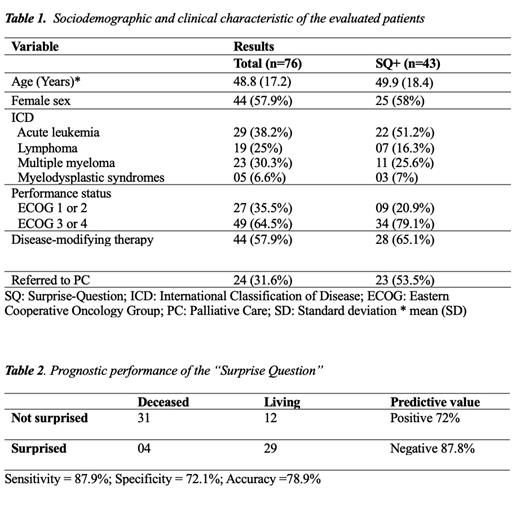
Results: During the study period, 76 patients were included after exclusion criteria (age<18 and not HM) were applied. Median age was 48.8 years-old (SD 17.2), and most participants were female (58%). Regarding the diagnosis, 38.2% had acute leukemia, 25% had R/R (refractory/relapsed) lymphoma, and 30.3% had R/R multiple myeloma. Forty-nine patients (64.5%) had poor performance status (ECOG 3 or 4), and most patients (57.9%) were receiving disease-modifying therapy. Physicians responded “No, I would not be surprised” (positive, SQ+) for 43 (57%) patients. After 12 months, 35 patients (46%) had died, from which 31 (89%) were in the group SQ - “No”. The sensitivity of the SQ was 88%, and specificity was 72%. The positive predictive value was 72%, negative predictive value was 88%, and accuracy was 79%. Notably, although clinicians found that 43 (57%) patients had a life expectancy of less than 12 months, only 24 (31.6%) were being followed-up with PC at the time of the interview.
Conclusion: The SQ has been shown to correctly estimate death among 68.3% of patients with blood cancers in the previous literature. In our study, the SQ demonstrated high sensitivity and potential clinical use. However, even within a center with full availability of a PC team dedicated to patients with advanced diseases and high morbidity and mortality, such as acute leukemia, the hematology team had rarely referred patients to PC. Despite advances on the importance of PC in the care of patients with HM, there is still an important delay when requesting the involvement of this specialty. Thus, effective strategies in order to integrate PC within this scenario are crucial.
Disclosures
Crusoe:Amgen, BMS, Janssen, Sanofi, Takeda: Honoraria; Janssen: Research Funding. Salvino:Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; OrphanDrugs: Consultancy; Abbvie: Consultancy, Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; ABHH: Membership on an entity's Board of Directors or advisory committees; SBTMO: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal